Information for laboratories contributing data to the UK Research and Innovation (UKRI), Data to Early Diagnosis and Precision Medicine Centre of Excellence (D2EDPM CoE) Interoperability workstream on Quality Assurance
Why should we get involved?
There is consensus that quality assurance is a crucial aspect in any pathology laboratory but digital whole slide imaging (WSI) is a relatively new technology and quality measures and metrics for this are not yet fully defined. We know staining can induce colour variability, which results in digital variability when slides are converted to WSI. This survey will allow us to accurately describe and measure this variation nationally for the first time. Our objective is to collaborate with all the laboratories and partner trusts within the Centre of Excellence network, to accumulate and share this important data and to make sure our network is at the forefront of quality assurance within our field.
Who is invited?
All pathology laboratories and partner trusts who undertake H&E staining and are connected to a UKRI Centre of Excellence (Research or Clinical). If you have received this email, this will be you but please get in touch if you are not sure).
What do I need to do?
After the return of a proforma stating your interest in taking part in the staining survey to NPIC, a network of pathology labs and named quality coordinators will be formed.
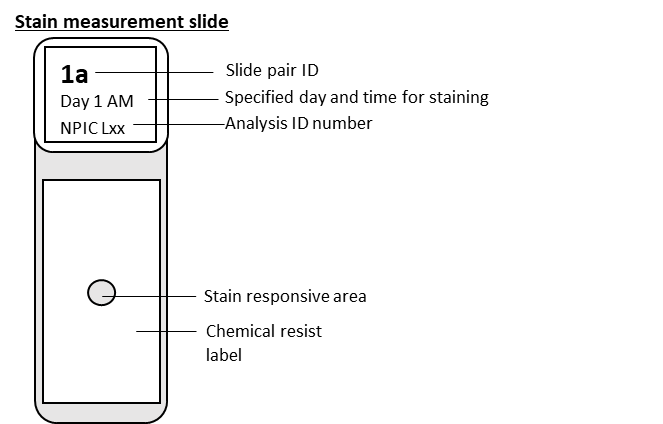
Stain quantification slides (Tango slides) will be posted via courier to you. ‘Tango slides’ are stained using your typical staining instruments and using your normal haematoxylin and eosin (H&E) staining protocol. The slides can be run alongside your normal tissue slides.
To be carried out over a period of approximately two weeks:
No slide preparation or special treatment needed.
20 slides stained with H&E over a period of 10 days (Monday to Friday). One slide to be ran each morning and one each afternoon.
A questionnaire will be supplied alongside stain quantification slides asking questions about the staining of the Tango slides and the staining protocol carried out.
NPIC will organise return of the sides via courier collection after staining.
What will happen to the data we provide?
An anonymised report will be issued back to each lab summarising all contributed data, only your data will be identifiable to you.
No data that can be used to identify individual labs will be shared publicly or with other centres within the workstream.
Improving the quality of WSI is an iterative process and the first steps are just to collect information.
Hence the data set needs to include variability and reflect ‘real life’ staining across the network.
These tools are not intended to mandate quality thresholds across the network.
Anonymised summary data will be presented to the network members and board CoE members later in the year.
What if we have more questions?
The stain survey is being led and co-ordinated via the NPIC Quality Coordination Centre at Leeds Teaching Hospitals NHS trust.
CONTACT FOR QUESTIONS:
Lead BMS: Clementine Hadcock – clementine.hadcock@nhs.net
Project Lead: David Brettle – davidbrettle@nhs.net
